From the start of the COVID-19 pandemic, an enormous amount of information has been communicated about the virus and how to keep ourselves and our workplaces safe.
COVID-19 vaccines can stop the spread by helping you not get sick, but whether you are an employer, employee or a patron of a business there are often more questions than answers.
Here are some answers to commonly asked questions about COVID-19 vaccines provided by the Canadian Centre for Occupational Health and Safety.
There are often more questions than there are answers when it comes to COVID-19 vaccines and if you did not find the resources that you are looking for, please visit our other informational posts in the Vaccine 101 for Business section or contact us with your questions and we will support you in any way that we can.
What vaccines are currently available in Canada?
Do these vaccines cause COVID-19?
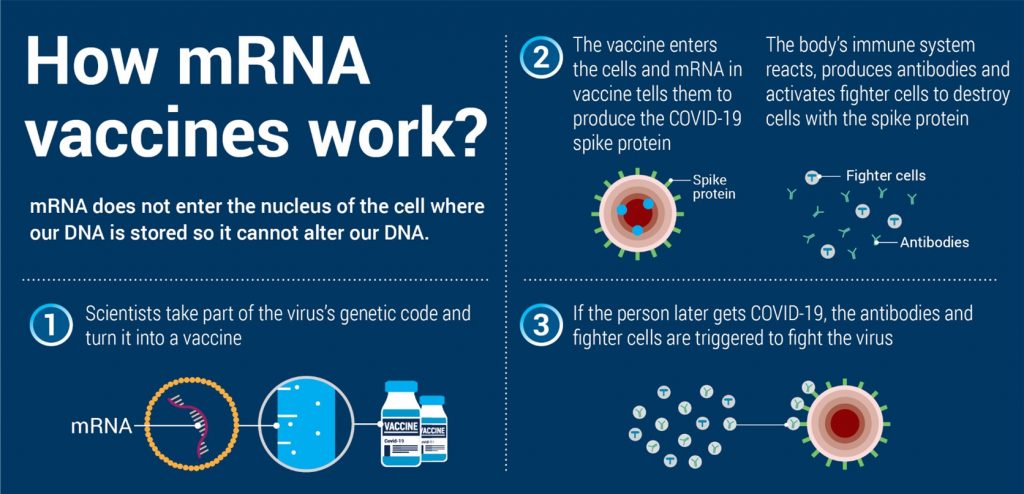
How do the vaccines work?
The Pfizer-BioNTech and Moderna COVID-19 vaccines are mRNA (messenger RNA) vaccines. They provide instructions to your cells to make a protein that is found on the COVID-19 virus.
The AstraZeneca COVID-19 vaccine and the Johnson and Johnson COVID-19 vaccines are viral-vector based. They use a harmless virus (e.g., an adenovirus like one that causes the common cold) as a delivery system. The vector virus contained within this vaccine produces the SARS-CoV-2 spike protein. This protein is found on the surface of the virus that causes COVID-19. This protein will not make you sick. It does its job and goes away.
All four vaccines work in a similar way. Our immune system knows that the protein doesn’t belong in our body and begins making immune responses, including antibodies. These immune responses will help fight the virus if you get exposed to COVID-19 in the future.
Will the vaccines work on the new variants?
Do the mRNA vaccines contain preservatives, adjuvants, or animal products?
No. Some vaccines can contain adjuvants (for example, aluminum salts) to help boost the body’s response to the vaccine, antibiotics to help prevent contamination during the manufacturing process, and preservatives and stabilizers to keep the vaccine stable, effective and safe when it’s being made, shipped and stored. The mRNA vaccines do not contain any of these products. There are no animal or human materials in the mRNA vaccines. They also do not contain any latex.
Learn more about the Moderna vaccine, the Pfizer-BioNTech vaccine, and the AstraZeneca vaccine. Get more information on authorized vaccines for COVID-19.
What is polyethylene glycol (PEG)?
Both the Pfizer-BioNTech and Moderna vaccines contain polyethylene glycol (PEG). PEG is a potential allergen. It is also used in bowel preparation for colonoscopies, laxatives, cough syrup, cosmetics, contact lens solutions, skin care products and as an additive in some food and drinks.
The AstraZeneca vaccine does not contain PEG but does contain a potential allergen, polysorbate 80. It is used in cosmetics and in medical preparation (e.g., vitamin oils, tablets).
Consult with your health care provider or allergist to discuss whether vaccination is right for you.
Can I still receive the vaccine if I have allergies?
Are there other medical conditions to consider before getting vaccinated?
Yes. COVID-19 vaccinations might be prioritized for those with underlying medical conditions who may be at risk for more severe disease or outcomes of COVID-19 (e.g., older adults and people who are living with obesity).
Anyone with questions or concerns, including those who are pregnant or breastfeeding, or those who are immunocompromised (e.g., a reduced ability to fight infections or other diseases) should consult with their health care provider about the risks and benefits of COVID-19 vaccination. The National Advisory Committee on Immunization has prepared recommendations on the use of COVID-19 vaccines. The Public Health Agency of Canada has a tool kit for health care providers.
How many doses do I need?
Can I take a different vaccine for my second dose?
What are the possible side effects from these vaccines?
Mild to moderate side effects are very common and last no more than a few days. Common side effects include pain at the injection site, tiredness, headache, muscle pain, joint pain, chills, and fever.
A serious adverse event following immunization such as a severe allergic reaction (e.g. anaphylaxis) is very rare. Some side effects, like fever, happen more often after the second dose.
Ask your health care provider what they recommend to help with any potential side effects.
How soon can I get vaccinated?
COVID-19 vaccines are available to everyone in Canada who is eligible to receive a COVID-19 vaccine and the available vaccine supply will be distributed in phases.
Priority populations were identified for early immunization due to the limited and staggered arrival of vaccine supply.
Initially, the vaccine was given to persons living and working in congregate living centres that provide care for seniors (e.g., long-term care, assisted living, chronic care, and retirement homes); adults aged 70 years and older, beginning with those aged 80 years and older; health care workers and personal support workers providing direct patient care; and adults in Indigenous communities.
As vaccine became more readily available it has been given to health care workers not included in the initial rollout, residents and staff of all other congregate settings (e.g., migrant workers, correctional facilities, homeless shelters), and essential workers (e.g., police, firefighters, food production).
Vaccinations will continue to expand to all Canadians and eligibility requirements vary by province. To learn more about the Manitoba vaccine roll-out, please click here.
How long will I be protected once I receive the vaccine? Will I need one annually?
Can my employer make COVID-19 vaccination mandatory?
I had COVID-19. Do I still need to get vaccinated?
How many people need to get the vaccine for herd immunity to work?
I’ve been vaccinated. Do I still need to wear a mask?
Yes. Currently there isn’t enough scientific evidence to say whether the spread of COVID-19 can be stopped by vaccination alone. Because of this, it is essential that everyone continues to follow public health measures to help stop the spread of COVID-19.
It’s important to avoid crowds and crowded spaces, keep at least 2 metres away from people outside of your household, wear a well-constructed and well-fitted mask that covers your nose, mouth, and chin, and practice good hand and respiratory hygiene. Wash your hands often with soap and warm water for at least 20 seconds or use a hand sanitizer containing at least 60% alcohol.
Cough or sneeze into a tissue or the bend of your arm, not your hand. Dispose of any tissues you’ve used as soon as possible in a lined waste basket and wash your hands immediately afterwards. Avoid touching your eyes, nose, or mouth with unwashed hands.
It is important that mental health resources and support are provided to all workers, including access to an employee assistance program, if available.
For further information on COVID-19, refer to the Public Health Agency of Canada and sign up for the Manitoba Business Matters newsletter to receive our updates.
Disclaimer: As public and occupational health and safety information is changing rapidly, local public health authorities should be consulted for specific, regional guidance. This information is not intended to replace medical advice or legislated health and safety obligations. Although every effort is made to ensure the accuracy, currency and completeness of the information, CCOHS does not guarantee, warrant, represent or undertake that the information provided is correct, accurate or current. CCOHS is not liable for any loss, claim, or demand arising directly or indirectly from any use or reliance upon the information.